


IBT specializes in delivering top-notch reagents tailored for research and development purposes.
By Lucy Greenfield & Shweta Kailasan
Given the staggering global impact of seasonal influenza, affecting up to one billion individuals annually and millions in the U.S., the urgent development of effective influenza vaccines, particularly a universal flu vaccine, is paramount due to the virus’s constant evolution through antigenic drift and shift. To accurately assess the efficacy of these novel vaccine candidates, including those leveraging mRNA vaccine technology, recombinant proteins, and nasal spray formulations targeting conserved regions of the hemagglutinin (HA) protein, the hemagglutination inhibition (HAI) assay remains a crucial and cost-effective benchmark, as it is the only recognized assay for measuring virus-neutralizing antibodies and predicting vaccine-induced immune protection.
Given the staggering global impact of seasonal influenza, affecting up to one billion individuals annually and millions in the U.S., the urgent development of effective influenza vaccines, particularly a universal flu vaccine, is paramount due to the virus’s constant evolution through antigenic drift and shift. To accurately assess the efficacy of these novel vaccine candidates, including those leveraging mRNA vaccine technology, recombinant proteins, and nasal spray formulations targeting conserved regions of the hemagglutinin (HA) protein, the hemagglutination inhibition (HAI) assay remains a crucial and cost-effective benchmark, as it is the only recognized assay for measuring virus-neutralizing antibodies and predicting vaccine-induced immune protection.
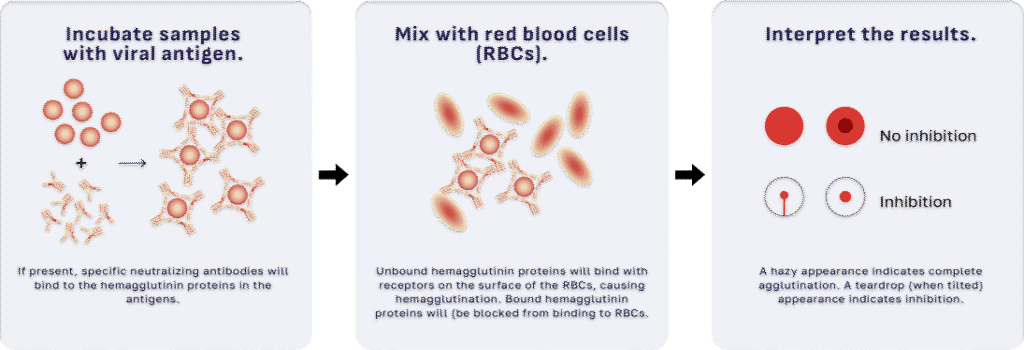
An assay plate following an incubation with influenza and the serum sample. These methods are essential not only for virus characterization but also for identifying new antigenic variants, vaccine strain selection, and sero-epidemiologic studies of influenza virus transmission and prevalence. Here’s a concise explanation of the assay’s mechanism:
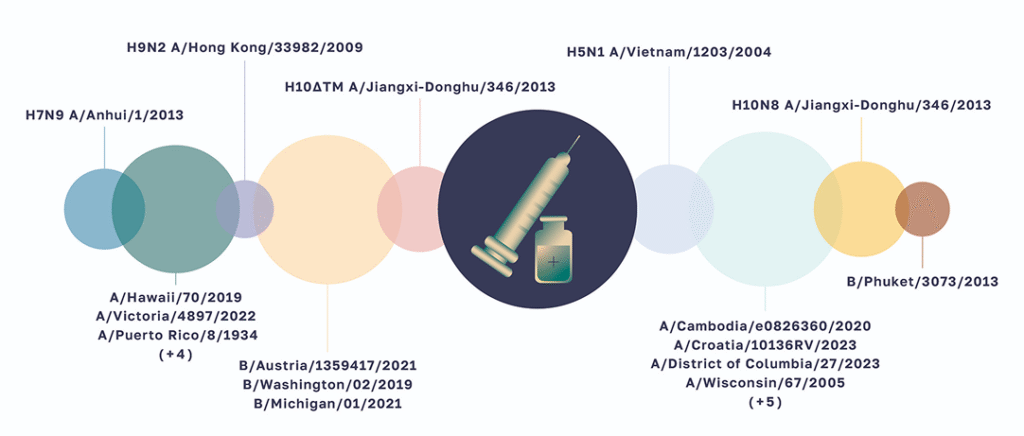
At the heart of our HAI assay lies our ability to work with a diverse range of antigens, the viral markers that trigger your immune response. The following types of antigens can be utilized:
The dynamic, replicating form, revealing the virus in its active state.

The safe, non-replicating form, providing a stable snapshot of the viral target.
Engineered for enhanced replication in mice.
Pure, isolated Hemagglutinin protein for highly specific assays.
Each antigen offers a unique window into the immune response, allowing us to capture a comprehensive picture and IBT Bioservices has been successful in setting up the HAI assay using various types of antigens.
Each antigen offers a unique window into the immune response, allowing us to capture a comprehensive picture and IBT Bioservices has been successful in setting up the HAI assay using various types of antigens.
Achieving assay harmonization in HAI testing can be facilitated by adopting consensus protocols. Additionally, having the right biological standards offers a viable solution to standardize assays and translate readings into normalized values or international units (IU), ensuring comparability across studies and among different laboratory methodologies. Hence, the WHO method to standardize the amount of viral particles that are added to each sample is the preferred method ensuring plate-to-plate comparability within an assay, but between assays as well. Gaining this insight is vital for navigating the market and driving product success.
2023-2024 U.S. Flu Season: Preliminary In-Season Burden Estimates | CDC
Kumar, Arun & Meldgaard, Trin, Bertholet, Sylvie. (2018). Novel Platforms for the
Development of a Universal Influenza Vaccine. Frontiers in Immunology. 9. 600.
10.3389/fimmu.2018.00600.
Influenza Virus. Methods and Protocols. Springer Protocols. Humana Press. 2018
Subscribe to the IBT Bioservices Tech Talk list and receive periodic updates on industry news and IBT Bioservices products and services.