


IBT specializes in delivering top-notch reagents tailored for research and development purposes.
A Microneutralization (MN) Assay is used to measure the ability of serum or antibodies to neutralize the infectivity of a virus. This assay is a crucial tool in virology and immunology for assessing the presence and potency of neutralizing antibodies in biological samples.
At IBT Bioservices, we provide microneutralization assay services designed to deliver precise and reliable data for vaccine & therapeutic development, immunology research, and diagnostics. With a team of experts and access to a broad range of viral strains, we offer fully customized solutions tailored to your specific research goals. We maintain rigorous quality control and use state-of-the-art technology, ensuring the highest standards for every assay.
At IBT Bioservices, we follow a well-defined process to ensure accuracy and efficiency in our microneutralization assays:
In addition to microneutralization assays, we offer Influenza ELISA services. ELISA (Enzyme-Linked Immunosorbent Assay) is used to measure the concentration of specific antibodies against influenza viruses, providing important data on immune response and vaccine efficacy. Our Influenza ELISA services are an integral part of our virology research offerings, helping clients gain insights into influenza immunity and antibody titers.
We have a large panel of strains available for testing, including:
We also offer custom assay development for emerging pathogens, ensuring you stay at the forefront of vaccine and antiviral research.
Plates were seeded with MDCK cells one day prior to the assay. Antibody 1, Antibody 2, and the Internal Control were serially diluted to Concentrations 1-8 and incubated with Influenza B/Brisbane/60/2008 at a predetermined MOI. The antibody/virus mixture was added to the cells and incubated at 35°C for several days until sufficient cytopathic effect (CPE) was observed in the Virus Only control wells. Cells were stained with crystal violet. Viable cells stain bright purple.
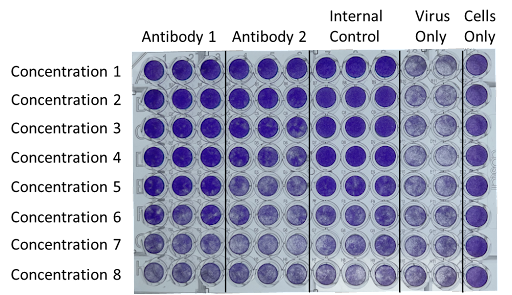
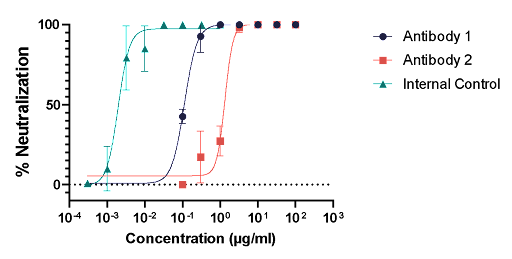
The Microneutralization Assay is based on the principle that neutralizing antibodies have the capacity to inhibit or block the ability of a virus to infect host cells. By measuring the reduction in viral infectivity in the presence of specific antibodies, researchers can determine the neutralizing capability of these antibodies.
These assays are invaluable tools for understanding the immune response to viral infections and the assessment of vaccines. They provide important information about the presence and potency of neutralizing antibodies, which are crucial for protection against many viral diseases.
Microneutralization Assays are used to study the immune response to viral infections, vaccine efficacy, and the development of immunity in individuals or populations.
These assays play a critical role in evaluating the effectiveness of vaccines by assessing their ability to induce neutralizing antibodies.
MN assays are employed in seroepidemiological studies to estimate the prevalence of specific antibodies within a population, providing insights into the history and prevalence of viral infections.
Some MN assays are used for diagnostic purposes, especially for confirming the presence of antibodies to specific viruses, such as in the case of diagnosing certain viral diseases like measles or mumps.

Our team of scientists and technicians are highly skilled and experienced in conducting HAI assays, ensuring accurate and reliable results.

We understand the unique needs of each research project and provide customized assay designs to meet your specific requirements.

We prioritize efficiency without compromising on quality. Our streamlined processes and advanced equipment allow for quick turnaround times, ensuring you receive results promptly.

We maintain rigorous quality control measures throughout our operations, ensuring the accuracy and reproducibility of our assay results.

Our dedicated customer support team is always available to address your queries and provide assistance at every step of the process.
Here are some of the most frequently asked questions to help you out with any questions you may have.
A NAb (Neutralizing Antibody) assay measures the ability of antibodies to neutralize a virus by inhibiting its infectivity in vitro. The assay provides crucial information about the potency of antibodies, which is especially important in evaluating vaccine responses and immune protection.
We most commonly use MDCK, Vero & A549 cells for microneutralization assays, but other cell types may be used depending on the pathogen being studied. The selection of cell type depends on the host specificity of the virus.
PRNT (Plaque Reduction Neutralization Test) and MNT (Microneutralization Test) are both neutralization assays, but they differ in methodology. PRNT measures the reduction of viral plaques (clear zones of cell lysis), while MNT measures the reduction of cytopathic effects (CPE) in host cells. MNT is generally faster and more scalable for high-throughput testing compared to PRNT.
Contact us today at services@ibtbioservices.com to learn more about our Microneutralization Assay Services and how we can support your research in virology, immunology,vaccine or therapeutic development.