


IBT specializes in delivering top-notch reagents tailored for research and development purposes.
| Animal Background: | Age Ranges: | Inoculations: | Therapeutic Agents: |
|---|---|---|---|
Mice, Rats, Cotton rats, Guinea Pigs, Hamsters |
Neonates to elderly |
BSL-2 pathogens, peptides, small molecules, etc. |
antibodies, small molecules, adjuvant mediated, new constructs, etc. |
| Housing: | Treatment Administration Routes: | Readouts: | Regulation: |
Specialized housing for Immunocompromised mice, double filter Individual ventilated cages and racks |
Intravenous, Intramuscular, Subcutaneous, Footpad, Intradermal, Oral gavage, Retro-orbital, Nebulizer, Sublingual |
Animal health, weight, and survival data, CPE Assay, HAI Assay, PRNT, Microneutralization assay, Pseudovirus neutralization assay, ELISA, Luminex, Flow |
Attending veterinarian, engaged IACUC committee |
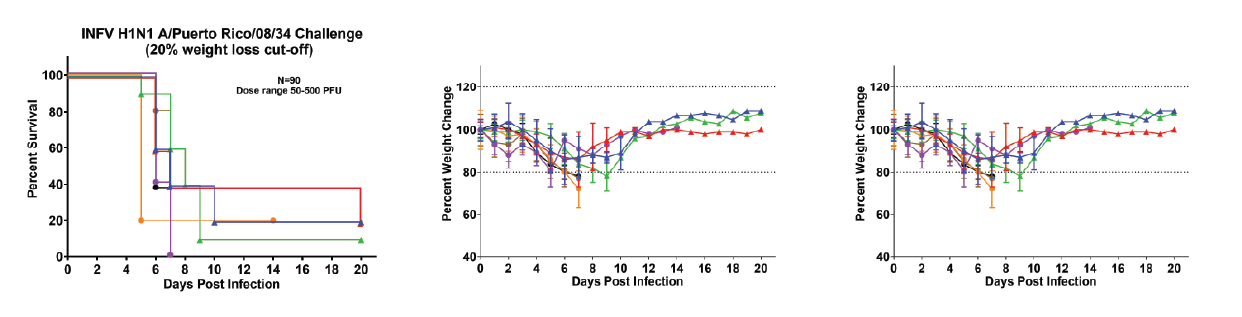
Description: INFV H1N1 A/Puerto Rico/08/34 dose determination study in BALB/C mice when treated with 20mg/kg of oseltamivir phosphate twice daily for five days. Meta-analysis of seven independent studies (n=45).
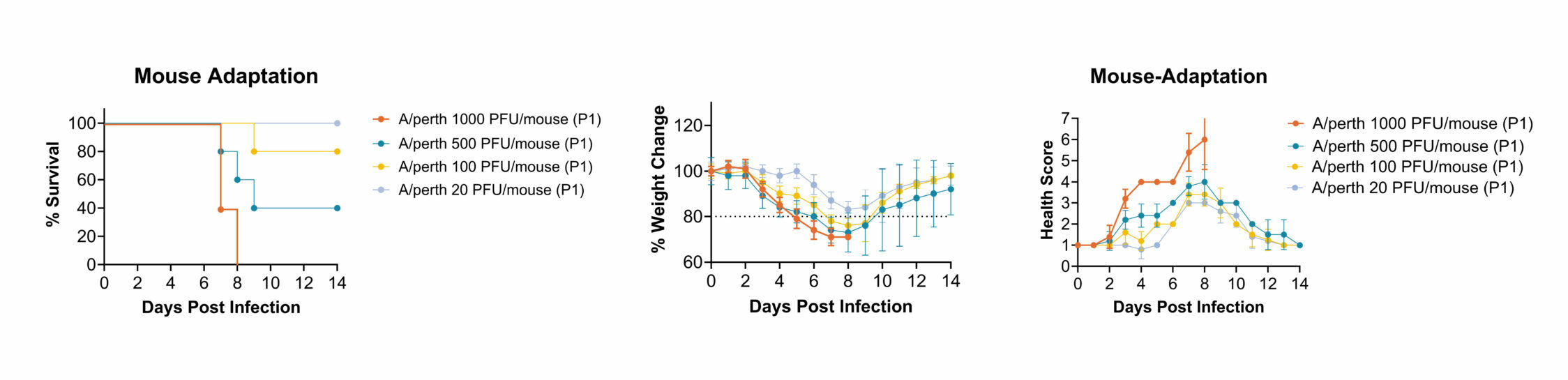
Description: 10 mice were inoculated via intranasal instillation with 1.00E+05 PFU/mouse of A/Perth/261/2009 MH25Y (oseltamivir phosphate resistant strain). On day 4 post-infection, all animals were sacrificed, and lungs were harvested. Lungs were homogenated and final supernatants were titrated via plaque assay. The passage 1 (P1) lung supernatants were then used to infect new BALB/C mice.