


IBT specializes in delivering top-notch reagents tailored for research and development purposes.
Tap into our off-the-shelf antiviral assay testing services to screen for anti-viral activity of your compounds against key virus families or let us help you select the appropriate assays for your specific compound and mechanism of action.
Our anti-viral assays are designed to evaluate the activity of your compound of interest. In addition to anti-viral activity, any of these assays can be further optimized or used in combination with complementary assays to determine mechanism of action. Moreover, complement these assays with our cytotoxicity
assays to ensure the anti-viral activity of your compounds are meaningful and within a reasonable therapeutic window. IBT also has quality systems in place to adapt our assays for clinical sample testing. Explore the multitude of anti-viral assays offered by IBT Bioservices below
IBT BioServices stands out as a leader in antiviral testing services. Our expertise in antiviral assays enables us to deliver tailored, precise, and compliant results. We utilize advanced antibody antiviral screening, virus panels, and compound screening assays to support a wide range of research needs. By partnering with IBT, you gain access to a team of experts committed to accuracy and efficiency, ensuring that each antiviral testing process meets the highest industry standards. Our antiviral testing laboratory is designed to handle complex testing requirements, offering reliable and reproducible results for regulatory submissions and scientific studies.
Our antiviral testing process is streamlined to provide accurate, timely, and consistent results. From the initial consultation to the final report, we prioritize quality at every step. Utilizing cutting-edge antiviral in vitro assays and rigorous quality control measures, our team delivers dependable results that accelerate your research. Each sample undergoes thorough testing in our state-of-the-art antiviral testing laboratory, where we ensure adherence to regulatory standards. Our process is built to support high-throughput testing needs, from antibody antiviral screening to complex virus panels, making IBT the ideal choice for reliable antiviral testing services.
Our sample preparation protocol is designed to ensure the integrity and consistency of each antiviral assay. We accept a variety of sample types and offer detailed guidance on sample collection, handling, and submission to meet study-specific requirements. Following strict quality standards, we process samples efficiently and precisely, maximizing the reliability of antiviral in vitro assays and other testing methods. Clear guidelines are provided for sample volume, storage conditions, and preparation to ensure that every sample meets the necessary criteria for accurate antiviral testing.
At IBT, quality assurance is at the core of our antiviral testing services. Every assay, whether an antibody antiviral screening or compound screening assay, undergoes rigorous quality control to maintain high standards of accuracy. Our dedicated quality assurance team meticulously analyzes each data set, providing clear and reproducible results that adhere to regulatory standards. We employ robust data analysis techniques to ensure that each finding is credible, reliable, and suitable for supporting regulatory submissions and advanced research needs.
IBT BioServices utilizes state-of-the-art equipment and innovative technology for antiviral testing. Our antiviral testing laboratory houses advanced flow cytometry platforms, high-throughput screening systems, and specialized assay development tools. This technological foundation enables us to conduct precise antiviral in vitro assays, complex virus panel studies, and targeted compound screening assays with exceptional accuracy and speed. By staying at the forefront of technology, we ensure that our antiviral testing services meet the evolving demands of the scientific community.
Our antiviral testing services support a variety of applications across pharmaceutical research, clinical studies, and academic investigations. Common use cases include:
Vaccine Development: Providing essential data on viral response and antibody generation to support vaccine research.
Drug Efficacy Testing: Evaluating antiviral compounds’ effectiveness against various viruses to aid in drug discovery and development.
Regulatory Compliance: Offering compliant and detailed antiviral assay data to facilitate regulatory submissions.
Antibody Screening: Utilizing antibody antiviral screening to identify effective antibodies for therapeutic purposes.
Pathogen Research: Conducting virus panel studies to understand the behavior of emerging pathogens and assess therapeutic efficacy.
Our comprehensive range of antiviral testing services is designed to meet the diverse needs of researchers, helping drive advancements in treatment and prevention.
Ready to partner with IBT on advanced antiviral testing services? Schedule a consultation now to explore how our tailored testing solutions can support your antiviral research.
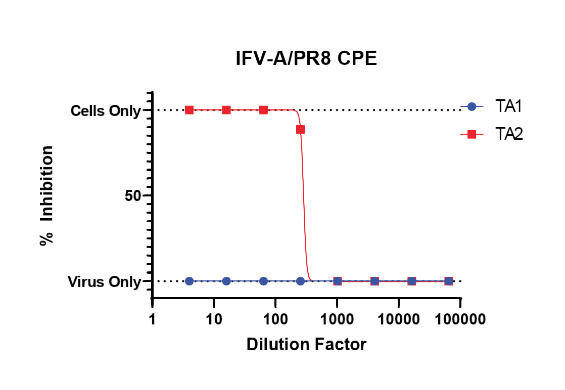
Our CPE inhibition assay can be used to assess a test article’s ability to hinder viral-induced cytopathic effect. This assay is ideal for compounds with mechanisms of action that impact the host cell.
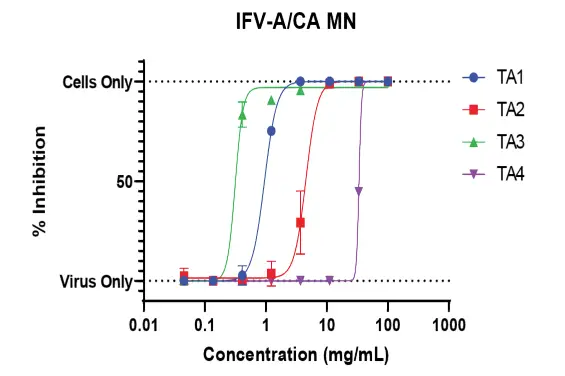
Another variant of the CPE assay is microneutralization, where the test samples are incubated and allowed to neutralize the virus before being incubated with cells.

Measuring an antiviral compound’s ability to decrease virus replication and subsequent viral yield in cell cultures using yield reduction assay. Following incubation, cell culture supernatants are collected, titrated via plaque assay & given as a titer (PFU/mL).
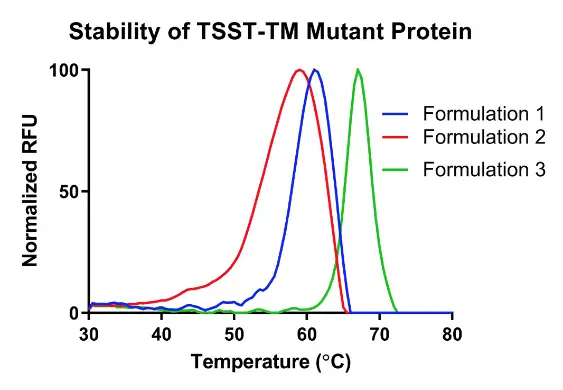
A high throughput stability-indicating assay to measure the melting temperature of your sample. The assay can be used to evaluate antibodies or proteins at varying formulations, stress conditions or stability timepoints that are generated over a temperature range of 30-100°C
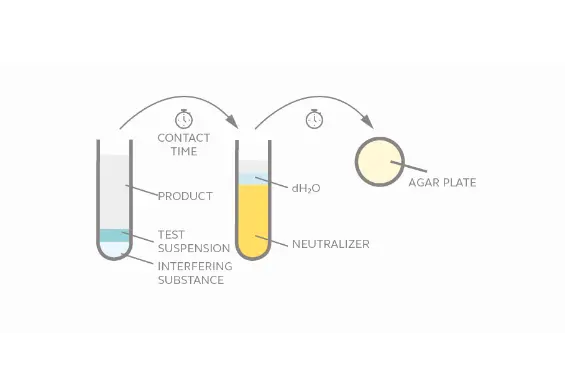
Evaluate virucidal activity of chemical disinfectants within a given contact time in suspension.
Measure the level of virus-specific neutralizing antibodies in a sample using PRNT assays, providing insight into the immune response against a particular virus.
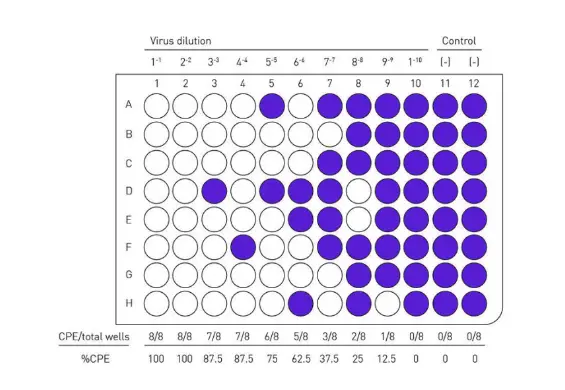
Evaluate TCID50, an endpoint dilution assay that can be used to calculate the viral titer of viruses by plaque assay. Plates are incubated until cytopathic effect (CPE) is observed in the virus control wells and then stained with crystal violet.

ADE has been observed in viruses such as Dengue and Influenza, and poses a challenge in vaccine development. This occurs when non-neutralizing or sub neutralizing anti-viral proteins facilitate virus entry into host cells leading to enhanced infectivity. Using flow cytometry, plaque assay or qPCR, this assay can help elucidate the ADE effect of test articles on virus infection in Fc receptor bearing cells.
Filovirus: Ebola virus Sudan virus Marburg virus Ravn Virus
Retrovirus: HIV
Henipavirus: Nipah Virus
Coronavirus: MERS-CoV SARS-CoV-2 SARS-CoV-2 Variants
Chikungunya Virus Dengue – Serotype 1,2,3 & 4 Human Cytomegalovirus (hCMV) Herpes Simplex Virus (HSV-1 & 2) Respiratory Syncytial Virus (RSV- A & B)
Influenza H1N1 Influenza H1N1 (Anti-viral resistant strains) Influenza H3N2 Influenza H3N2 (Anti-viral resistant strains) Influenza B (Yamagata Lineage/Victoria) Influenza B (Victoria Lineage)
What types of viruses can IBT test against?
IBT BioServices offers antiviral testing services against a wide array of viruses, including influenza, herpesviruses, coronaviruses, and emerging pathogens. Our antiviral in vitro assays are adaptable to meet the specific requirements of your study.
Are your antiviral assays compliant with regulatory standards?
Yes, our antiviral testing laboratory follows stringent regulatory standards to ensure accuracy and compliance, making our data reliable for regulatory submissions.
Can you assist with regulatory submissions?
Absolutely. Our antiviral testing services are structured to provide compliant and detailed data that can support your regulatory submissions, ensuring that your research is backed by robust, credible results.
How long does the antiviral testing process take?
The duration of antiviral testing varies based on the type of assay and virus. However, our process is designed for efficiency, and we aim to deliver results promptly without compromising quality.
What types of samples do you accept for testing?
Our antiviral testing laboratory is equipped to handle various sample types, allowing us to accommodate diverse research needs. Whether you’re conducting antibody antiviral screening or working with virus panels, we accept samples tailored to your testing requirements.
How do you ensure the accuracy and reliability of your test results?
Through a combination of advanced antiviral assays, rigorous quality control, and experienced personnel, we guarantee high standards of accuracy and reproducibility. Each test is carefully monitored to ensure reliability, helping you confidently advance your research.