


IBT specializes in delivering top-notch reagents tailored for research and development purposes.

Partner with our laboratory for GMP, GLP, and GCLP testing for your clinical trial needs and workflows. Our team has the regulatory and compliance know-how to guide you through the process. Equipped with powerful assays such as Luminex Testing, Neutralization Assays, HPLC Analysis, ELISA, and more, IBT can support all of your regulated testing needs.

We design and develop custom analytical methods to suit your unique product needs, ensuring compliance with FDA, ICH, and other global regulatory guidelines. Our experts have decades of combined experience in assay development, qualification, and validation on a variety of analytical platforms.

At IBT, we specialize in a range of compendial and non-compendial drug release methods to help you maintain the highest standards for quality, safety, and performance. Whether you’re developing a new formulation or adding a test to an existing product, our testing facility can deliver accurate and reliable results. IBT’s comprehensive range of GMP Release assays includes Sterility Testing (USP 71), Mycoplasma Testing(PCR Based), SDS-PAGE, Western Blot, A280 Measurements, and more.

We offer a range of conditions tailored to your product’s specific needs, from stability program design to ongoing stability testing, our team can handle every step. We can manage your stability programs over the entire lifespan of your product, providing you with actionable data to make informed decisions.

At IBT, we provide traceability and comprehensive documentation for every single sample from collection to transportation, storage, and processing. Our tracking system and temperature-controlled storage facilities offer the highest standards of quality and regulatory compliance.
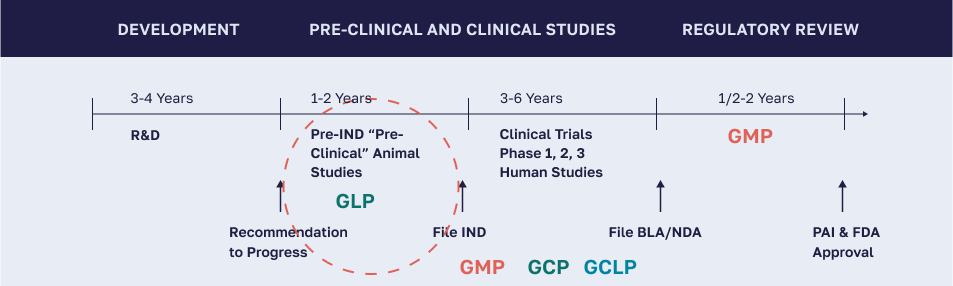
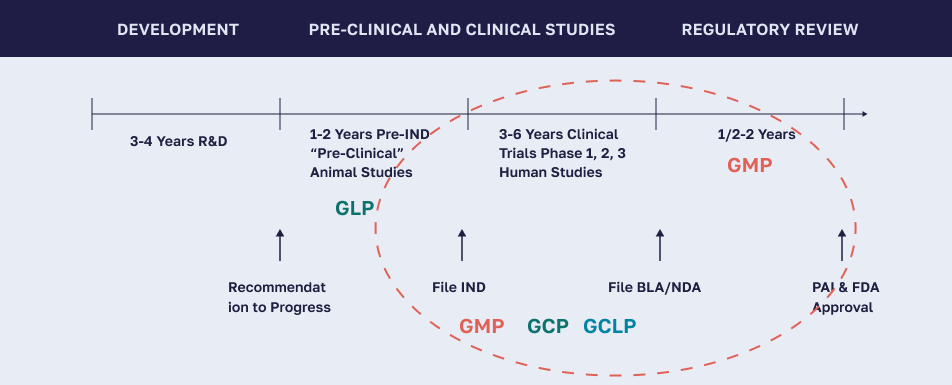
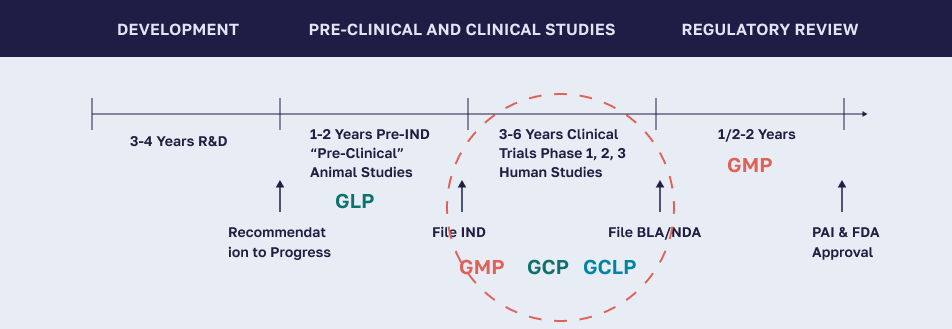
GLP is a set of conditions, processes, and documentation which support the conduct of nonclinical studies performed in a laboratory setting intended to assure the quality and integrity of data.

Master document for the conduct of the study. An outline of how the study will be conducted, including schedules, methods, and materials.

Characterization of the test articles under study, defining stability, storage conditions, potentially biohazardous cautions, and PPE needed in handling.

Summary of study results, capturing all outcomes and any deviations resulting from the study, Approved by the Study Director.

Luminex Testing, Neutralization Assays, HPLC Analysis, ELISA.
GMP is a set of conditions, processes, and documentation which support the production of drug candidates to quality and safety standards.

Master and executed batch records and instructions which document raw materials, processes, personnel, and deviations which result from the manufacturing process.

Physical, chemical, and microbiological tests to ensure the manufactured material meets pre-determined specifications, characteristics, and safety standards before released to market.

The review process by which all documents related to the production and subsequent testing of the manufactured material are examined and ensured to meet all regulatory and safety standards and subsequently transported to clinical sites to be administered in clinical trials.

Sterility Testing (USP 71), Mycoplasma Testing(PCR Based), SDS-PAGE, Western Blot,
A280 Measurements, Host Cell Protein Analysis (HCP Impurity), Bacterial Endotoxin / LAL Testing.
GCLP is a set of conditions, processes, and documentation which implement Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) principles to ensure the quality and reliability of clinical trial data generated by testing laboratories.

Establishing chain-of-custody of subject samples from the clinical site to the testing laboratory to both short-term and long-term storage and comprehensive documentation for all intermediate steps.

Overseeing facilities and equipment, ensuring use, maintenance, validation, and calibration are documented properly. Maintaining inventory control over all reagents and suppliers to guarantee the expeditious testing of samples.

Maintaining data integrity and traceability across all paper-based and digital documentation. Ensuring data is not subject to manipulation and bias and implementing a rigorous review process for all data generated.