


IBT specializes in delivering top-notch reagents tailored for research and development purposes.
| Animal Background: | Challenge Route: | Peak Lesion Size: | Standard Readouts: |
|---|---|---|---|
BALB/C, CD-1 |
Intradermal (ID) |
Strain dependent |
Weights, health, survival, lesions, photographs |
| Other Readouts: | |||

Description: Two groups of Balb/C mice were infected with 1.00E+07 CFU/mouse of Staphylococcus aureus USA300 (NRS384) on day 0 via the intradermal (ID) route. One group received the experimental vaccine while the other group served as infection only control. On Day 49 of the study, both groups were infected with 1.00E+08 CFU/mouse and monitored for an additional 14 days. Lesion areas of vaccinated and non-vaccinated mice after primary and secondary infection.
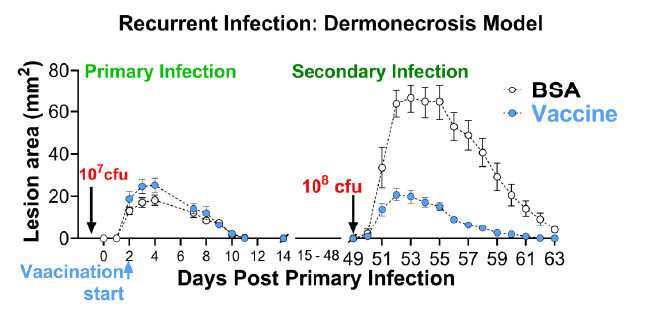
Description: Two groups of Balb/C mice were infected with 1.00E+07 CFU/mouse of Staphylococcus aureus USA300 (NRS384) on day 0 via the intradermal (ID) route. One group received the experimental vaccine while the other group served as infection only control. On Day 49 of the study, both groups were infected with 1.00E+08 CFU/mouse and monitored for an additional 14 days. Lesion areas of vaccinated and non-vaccinated mice after primary and secondary infection.
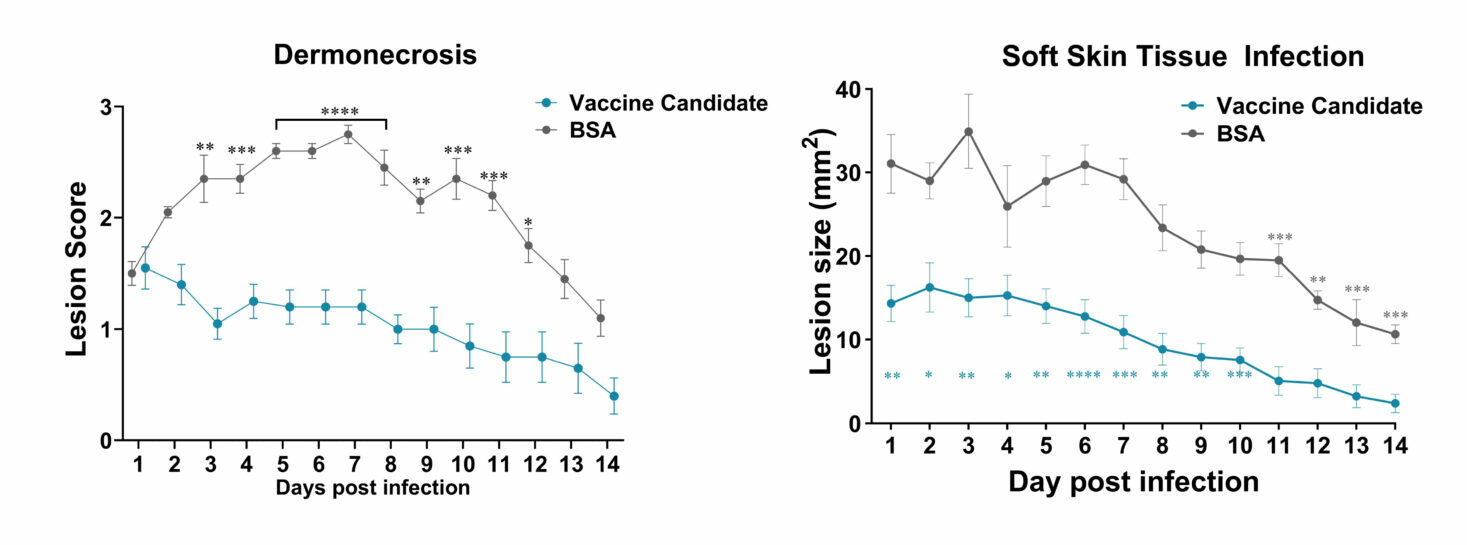
Description: Two groups of Balb/C mice were infected with 1.00E+07 CFU/mouse of Staphylococcus aureus USA300 (LAC) on day 0.
One group received a novel vaccine candidate while the other group served as control receiving BCA.