


IBT specializes in delivering top-notch reagents tailored for research and development purposes.

At IBT Bioservices, we offer SDS-PAGE services utilizing state-of-the-art equipment and
experienced staff to provide quantitative and qualitative analysis of proteins. Our SDS-
Page services offer a comprehensive analysis of purity, size, and integrity of a protein.
GMP Release Testing Available
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is a widely accepted and used technique for analyzing protein purity, size and integrity. The SDS compound, a surfactant, attaches to protein at a rate proportional to its molecular weight, imparting it with negative charges. Subsequent application of a negative charge allows for the separation of proteins on a gel based on molecular weight. Proteins are stained in a non-specific manner and are visualized as bands of varying density and size.
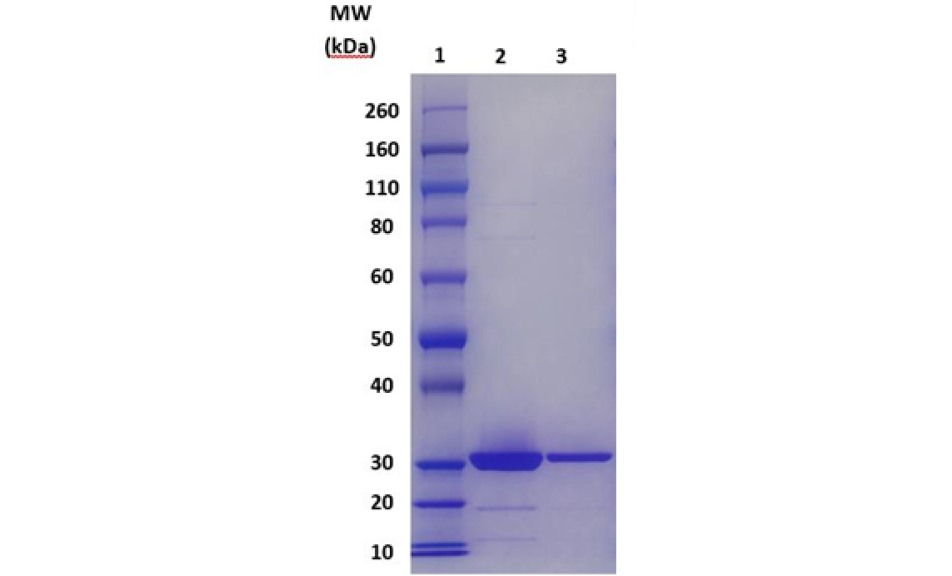
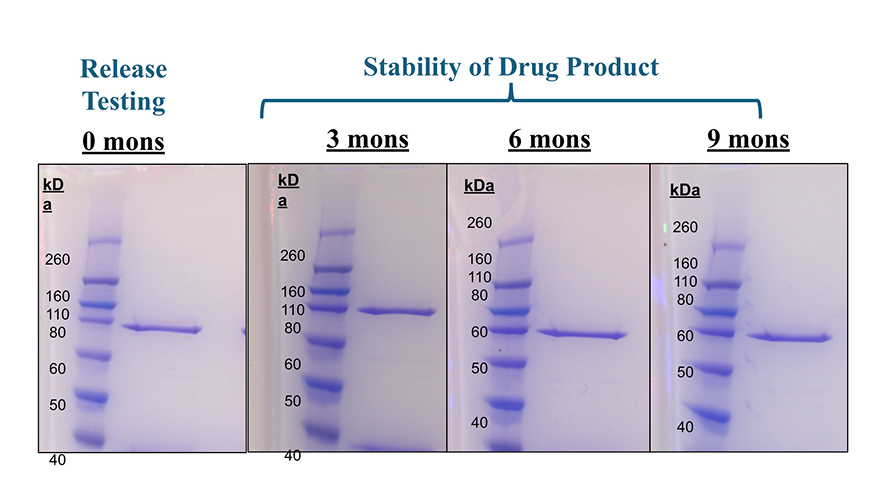
SDS-PAGE is a versatile analytical tool used in biopharmaceutical manufacturing for GMP release testing, serving multiple purposes: it assesses product purity by detecting and quantifying impurities such as host cell proteins, degradation products, or aggregates; confirms product identity by comparing molecular weight and banding patterns to reference standards; supports stability testing by revealing changes over time or under stress conditions; ensures the quality of raw materials like recombinant proteins before use; and monitors purification steps and validates manufacturing processes to maintain consistent product quality across different production lots.
We understand that every research project is unique. Our experts work closely with you to design customized Western Blot assays tailored to your specific requirements, ensuring optimal sensitivity and specificity.
Whether you need to analyze a large number of samples or perform a small-scale study, our laboratory is fully equipped to handle both high-throughput screening and low-volume testing. We offer quick turnaround times without compromising the quality and accuracy of the results.
We adhere to strict quality control protocols to maintain the integrity of our assays. Our team conducts regular internal quality assessments, and our processes are designed to meet or exceed industry standards, ensuring reliable and reproducible data.
Our services extend beyond performing the assay. We provide comprehensive data analysis and interpretation to help you make informed decisions. Our reports are clear, concise, and include all the necessary details, allowing you to easily understand and present your findings.
Our team of scientists and technicians are highly skilled and experienced in conducting HAI assays, ensuring accurate and reliable results.
We understand the unique needs of each research project and provide customized assay designs to meet your specific requirements.
We prioritize efficiency without compromising on quality. Our streamlined processes and advanced equipment allow for quick turnaround times, ensuring you receive results promptly.
Our dedicated customer support team is always available to address your queries and provide assistance at every step of the process.
We maintain rigorous quality control measures throughout our operations, ensuring the accuracy and reproducibility of our assay results.