


IBT specializes in delivering top-notch reagents tailored for research and development purposes.

Partner with our laboratory for GMP, GLP, and GCLP testing for your clinical trial needs and workflows. Our team has the regulatory and compliance know-how to guide you through the process. Equipped with powerful assays such as Luminex Testing, Neutralization Assays, HPLC Analysis, ELISA, and more, IBT can support all of your regulated testing needs.
With the ever-changing landscape, it is vital to keep up-to-date with industry guidance to ensure clinical trials are conducted in compliance with regulatory standards and expectations.
IBT offers technical expertise and state-of-the art GMP, GLP and GCLP testing facilities to guide you through all phases of your drug developmen journey. We ensure your products meet FDA, EU, and other international standards.

We design and develop custom analytical methods to suit your unique product needs, ensuring compliance with FDA, ICH, and other global regulatory guidelines. Our experts have decades of combined experience in assay development, qualification, and validation on a variety of analytical platforms.
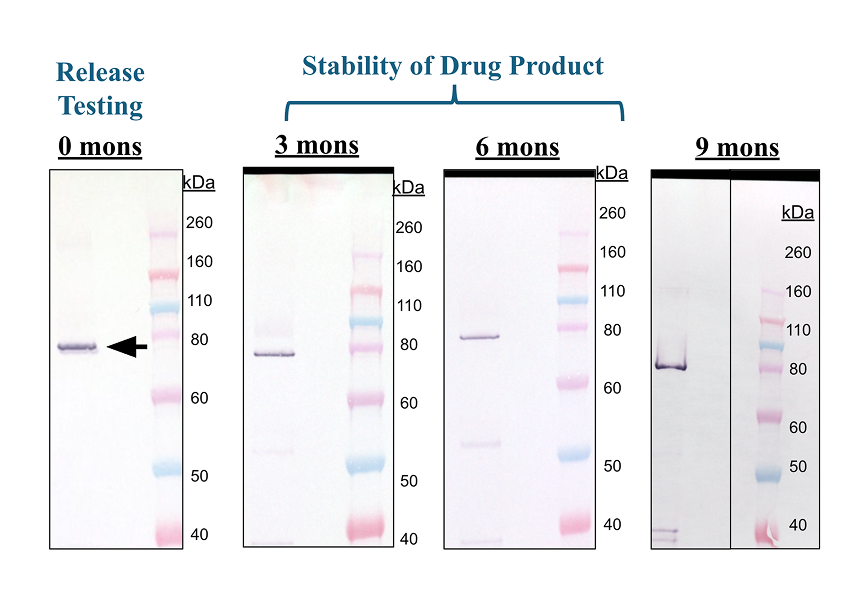
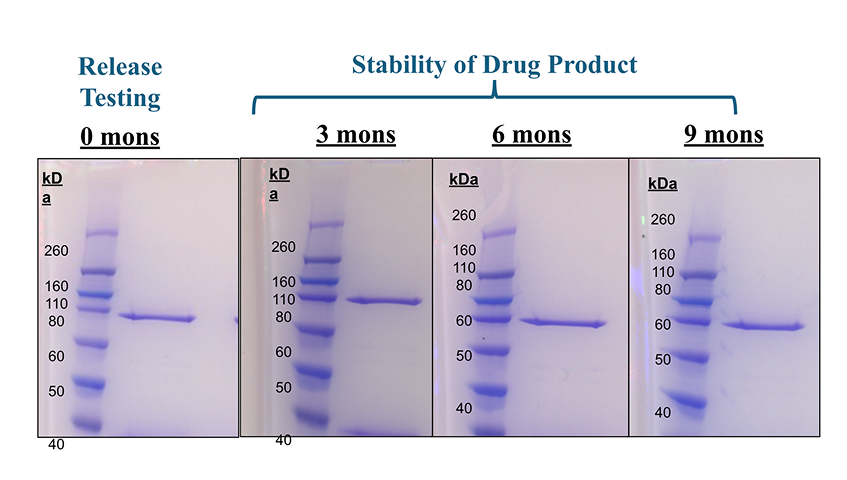
At IBT, we specialize in a range of compendial and non-compendial drug release methods to help you maintain the highest standards for quality, safety, and performance. Whether you’re developing a new formulation or adding a test to an existing product, our testing facility can deliver accurate and reliable results. IBT’s comprehensive range of GMP Release assays includes Sterility Testing (USP 71), Mycoplasma Testing(PCR Based), SDS-PAGE, Western Blot, A280 Measurements, and more.
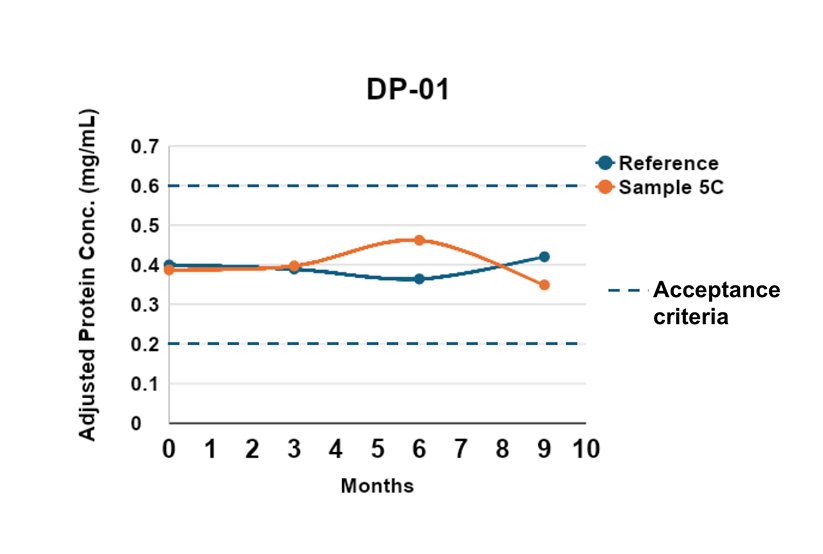
We offer a range of conditions tailored to your product’s specific needs, from stability program design to ongoing stability testing, our team can handle every step. We can manage your stability programs over the entire lifespan of your product, providing you with actionable data to make informed decisions.

At IBT, we provide traceability and comprehensive documentation for every single sample from collection to transportation, storage, and processing. Our tracking system and temperature-controlled storage facilities offer the highest standards of quality and regulatory compliance.
Our team of scientists and technicians are highly skilled and experienced in conducting HAI assays, ensuring accurate and reliable results.
We understand the unique needs of each research project and provide customized assay designs to meet your specific requirements.
We prioritize efficiency without compromising on quality. Our streamlined processes and advanced equipment allow for quick turnaround times, ensuring you receive results promptly.
Our dedicated customer support team is always available to address your queries and provide assistance at every step of the process.
We maintain rigorous quality control measures throughout our operations, ensuring the accuracy and reproducibility of our assay results.