


IBT specializes in delivering top-notch reagents tailored for research and development purposes.
Securing non-dilutive funding through prestigious programs like SBIR, STTR, NIH/NIAID grants and through contract mechanisms with agencies like DOD and BARDA is a monumental achievement for anyone developing innovative vaccines or modern therapeutics. These grants represent a crucial lifeline, providing the financial backing needed to propel groundbreaking research forward. However, the application process is highly competitive and demands a meticulously crafted proposal that demonstrates not only scientific merit but also a clear and feasible path to preclinical validation.
This is where identifying a reliable, one-stop preclinical Contract Research Organization (CRO) becomes a key differentiator and a significant factor in your grant success. Establishing such a partner not only offers logistical advantage, but it’s a strategic move that significantly strengthens your proposal by giving the reviewer confidence in your ability to execute the proposed plan within your timeline and budget.
IBT Bioservices has supported a number of companies, institutes, and individuals by providing technical insights to optimize research plans, cost estimates / letters of support for associated studies as well as timelines for critical milestones. Our tailored approach has allowed multiple clients to secure non-dilutive funding and federal grants, cumulatively totaling over $150M. Our team at IBT has supported 120+ clients to optimize grant applications for early-stage development by providing scientific advice, drafting study plans and providing cost estimates along with a letter of support.

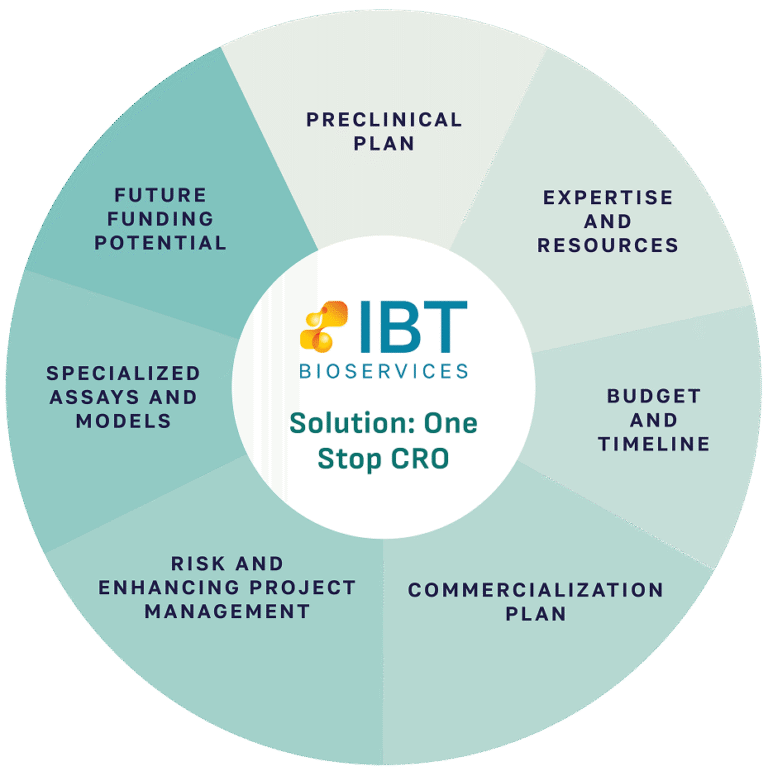
In summary, navigating the competitive landscape of SBIR, NIH, NIAID, BARDA or any other Funding Opportunity Announcements (FOA) requires a compelling scientific rationale coupled with a clear and credible preclinical development plan. Identifying a reliable, one-stop preclinical CRO like IBT Bioservices early in the application process is not just a logistical advantage; it’s a strategic move that significantly strengthens your proposal development.
Contact us today to learn how we can support your next grant application.
Our existing partners love boasting about our collaborations! We are happy to provide referrals if needed.
Subscribe to the IBT Bioservices Tech Talk list and receive periodic updates on industry news and IBT Bioservices products and services.