Chikungunya Virus
Our team at IBT Bioservices has extensive experience in preclinical testing services to assess the viability and effectiveness of novel therapies against arboviruses including chikungunya, dengue, and Zika viruses.
Caused by
Mosquitos, primarily Aedes aegypti and Aedes albopictus
Infects
Primarily humans
Symptoms
Fever, joint pain, muscle pain, headache, fatigue, rash
Virus Family
Arbovirus belonging to the Alphavirus genus of the Togaviridae family
Alphaviruses are arthropod-borne viruses that cause disease in humans and a wide range of domestic and agricultural animals. Interest in development of alphavirus therapeutics and vaccines has risen in recent years due to epidemics of Chikungunya virus (CHIKV) causing severe illness in Africa and Asia and concerns about possible use of Venezuelan equine encephalitis virus (VEE) as an agent of bioterrorism. We offer a diverse array of products and services desgined to facilitate alphavirus research, including chikungunya and Venezulan equine encephalitis viruses.
Our comprehensive range of Products includes
Anti-Chikungunya Antiserum
Purified polyclonal antibodies against Chikungunya
Venezuelan equine encephalitis antiserum and antibodies
Our preclinical testing services for arboviruses encompass a wide range of assessments, including:
Antiviral Assays
Using advanced cellular and molecular techniques, we evaluate the antiviral activity of potential drugs against the Chikungunya virus. These studies help identify compounds that can effectively inhibit viral replication, reduce viral load, and mitigate the disease’s progression. We investigate the mechanism of action of potential treatments and their impact on viral replication and host cell interactions.
Pharmacokinetic and Toxicology Studies
Understanding how potential treatments are absorbed, distributed, metabolized, and eliminated by the body is crucial. Our comprehensive pharmacokinetic studies provide valuable insights into the drug’s behavior, while toxicology evaluations ensure the safety of the treatment candidates.

ANIMAL MODELS
Our experienced researchers utilize appropriate animal models, such as mice or nonhuman primates, to mimic Chikungunya virus infection and assess the safety and efficacy of potential treatments. IBT has developed lethal chikungunya infections using Ag129 transgenic mice, who are deficient in IFN-α/β and IFN-γ receptors. With this capability, we are able to evaluate the efficacy of therapeutics against chikungunya using a rodent model. We closely monitor key parameters, including viral load, joint inflammation, immune response, and behavioral changes, to evaluate the effectiveness of the tested therapies. Our in vivo studies provide valuable data on the treatment’s ability to control viral replication, reduce disease severity, and improve overall outcomes. Animal Strains and Species: AG129 mice, BALB/c mice, C3h/hen mice
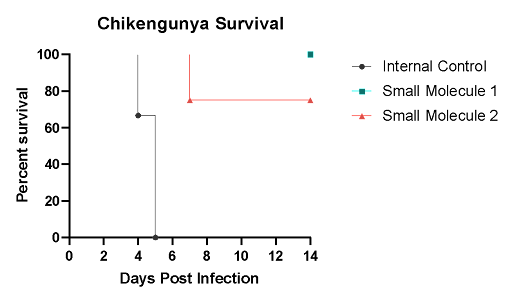
AG129 mice were challenged with 1.00E+04 of chikungunya virus CHIKV/181/25 via the intradermal footpad route. Mice were then treated daily via the intravenous (IV) route with either a small molecule therapeutic or 0.9% sterile saline. The mean time of death for this model is day 5.
At IBT Bioservices, we are committed to advancing the field of Chikungunya virus research and development through reliable and accurate preclinical testing services. Our dedication to scientific excellence, ethical practices, and adherence to regulatory guidelines positions us as a trusted partner in the fight against this global health challenge.Contact us today to learn more about how our in vitro and in vivo preclinical testing services can accelerate the development of promising treatments for the Chikungunya virus. Together, let’s make a difference in saving lives and securing a healthier future in the battle against this mosquito-borne viral infection.
REFRENCES
https://pubmed.ncbi.nlm.nih.gov/31697791/
https://pubmed.ncbi.nlm.nih.gov/34835037/